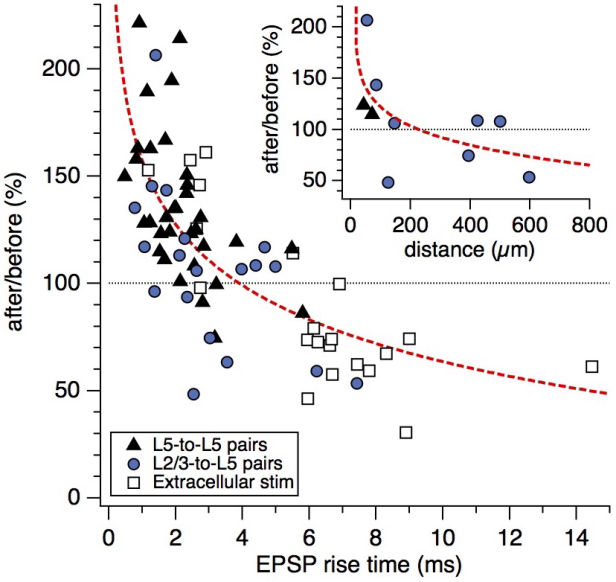
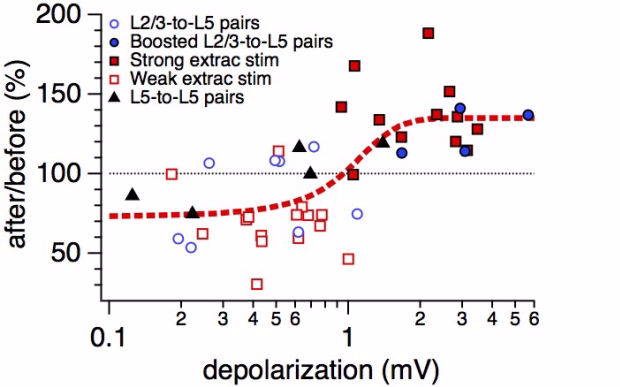
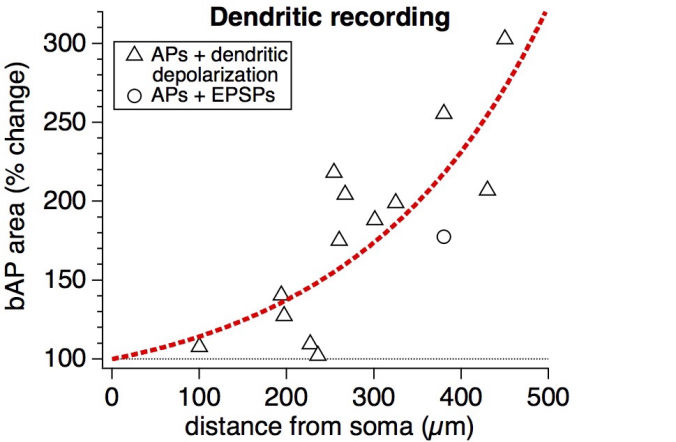
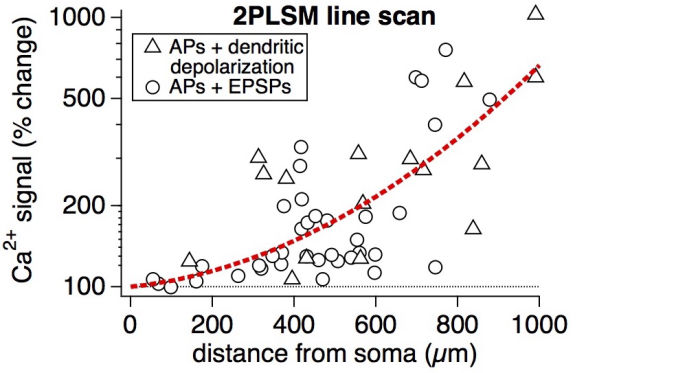
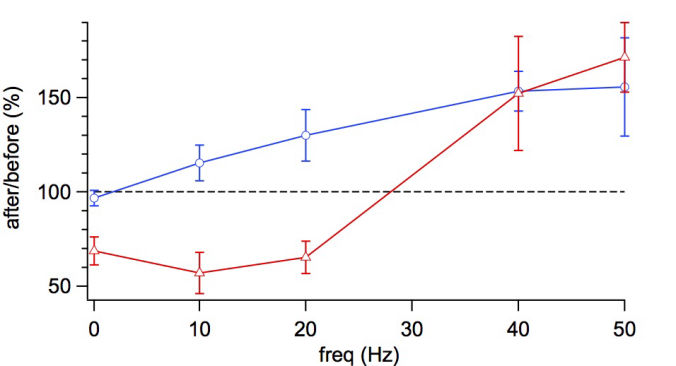
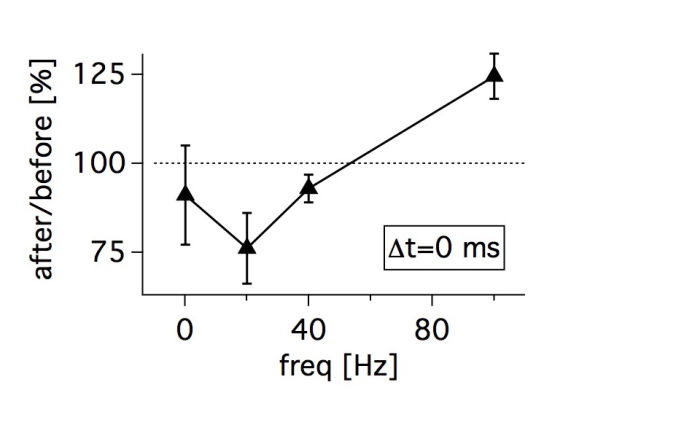
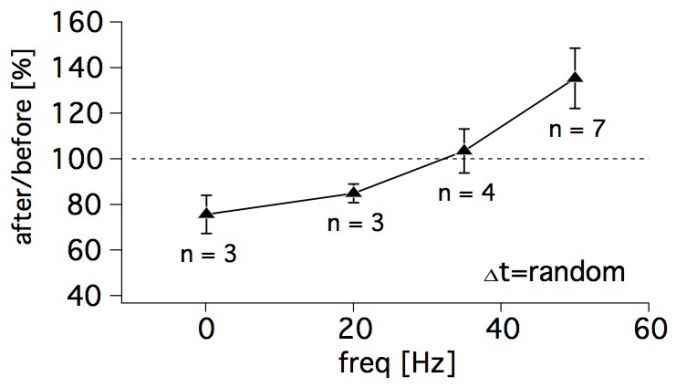
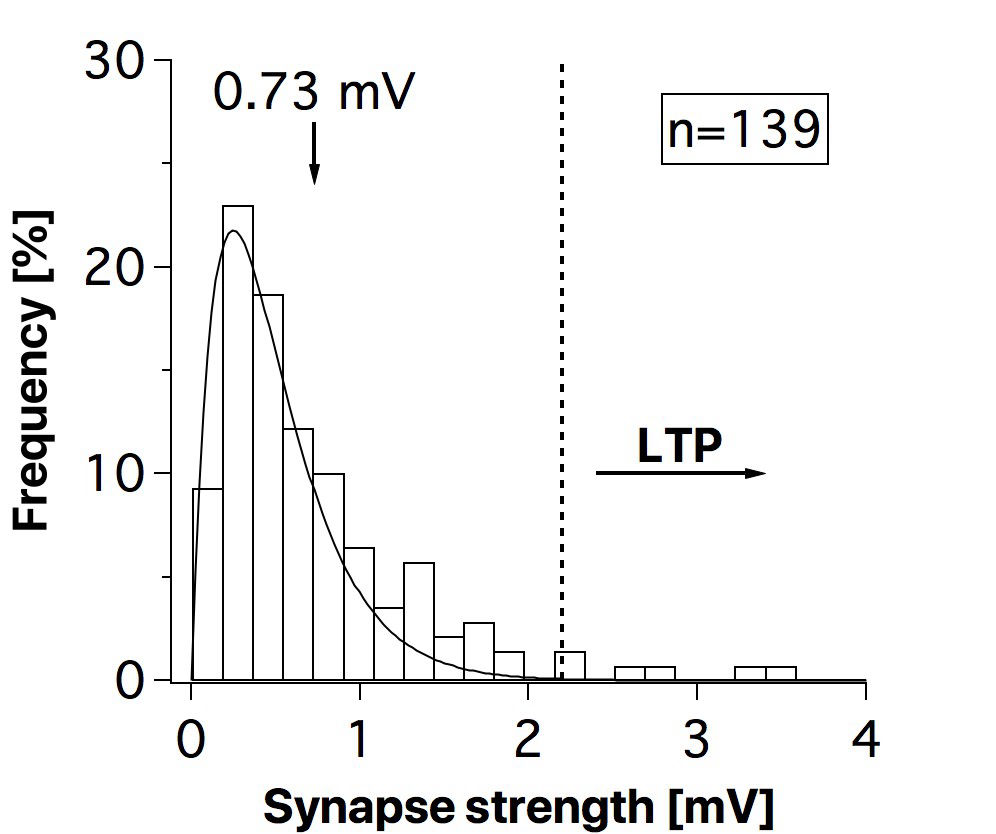
Connectivity datasetWith plasticity experiments using quadruple recordings, many cells are not connected. Many connections can also not be used for plasticity experiments, simply because the connections are very weak, or maybe they simply go bad. Fortunately, each quad recording provides a lot of data on the local connectivity. In the
Song et al, PLoS Biology 2005 study, we made use of a relatively large quad-recording data set generated this way, consisting of more than 900 connections and 8000 cells. The data is arranged as described below.
Format: Excel
Date: The date the experiment was conducted.
Attempt#: Each experimental day, I would attempt at getting a quad recording, and I would number each quad attempt.
nConnections: For each quad attempted, the nConnections parameter tells you how many connections I found.
nTested: For each quad attempt, I wouldn't necessarily get all four cells. If I got four, then I tested 12 connections, but if I only got three cells, then I tested 6 possible connections. With two cells, I tested two possible connections.
Age: The age of the animal, probably with a +/- one day slop or less.
CalciumConc: The concentration of calcium in the external solution. The reason this changes between 2.5 mM and 2.0 mM is because I modelled some of my studies on Feldman Neuron 2001 and Markram Science 1997. The former employed 2.5 mM, whereas the latter used 2.0 mM external calcium. The calcium concentration may affect both the amplitude and the CV of synaptic connections.
ConnectionString: Each quad recording has a ConnectionString associated with it, which uniquely identifies the connectivity pattern within the quad. For example, on 20/01/2001, Attempt #1, the string is 3_4,0.00020913,9.5405e-05; 4_3,0.00034122,0.00012075; 3_2,0.00050473,9.8416e-05; 2_3,0.00060293,0.00011659; 2_4,0.00060349,0.00013282; 1_4,0.0005404,0.00010957;. This means cell three was connected to cell 4 with a connective strength of 0.20913 mV and the standard deviation of that connection was 9.5404E-5 V. That pair also had a reciprocal connection from cell four to cell three, of 0.34122 mV strength, and so on and so forth. If the string says NA, then there was no connection found.
Note that all bad recordings were discared before this analysis stage, so recorded cells that were deemed bad were not included in the table -- this is important for obtaining correct connectivity counts.